Researchers at Stanford University and Department of Energy's SLAC National Accelerator Laboratory have made a pretty big breakthrough in lithium-ion battery technology. The team has developed a self-healing electrode using a stretchy polymer material that repairs cracks made in the electrodes caused by repeated use of the battery. This self-healing property could majorly extend the life of lithium-ion batteries in gadgets and electric cars.
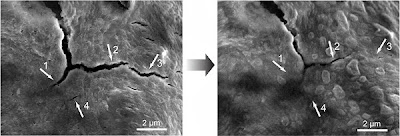
The university reports, "Silicon electrodes swell to three times normal size and shrink back down again each time the battery charges and discharges, and the brittle material soon cracks and falls apart, degrading battery performance. This is a problem for all electrodes in high-capacity batteries...To make the self-healing coating, scientists deliberately weakened some of the chemical bonds within polymers – long, chain-like molecules with many identical units. The resulting material breaks easily, but the broken ends are chemically drawn to each other and quickly link up again, mimicking the process that allows biological molecules such as DNA to assemble, rearrange and break down."
The electrodes coated with the polymer lasted 10 times longer than uncoated electrodes, which could make a huge difference in battery lifetimes.
"Their capacity for storing energy is in the practical range now, but we would certainly like to push that," said Yi Cui, an associate professor at SLAC and Stanford.
The coated electrodes worked for about 100 charge-discharge cycles before starting to significantly lose their energy storage capacity, which is still quite shy of the 500 cycles for cell phones and the 3,000 cycles for electric vehicles, but the researchers say the potential is there for getting those higher cycle numbers.
The team thinks that other electrode materials could work as well, but for now they're focusing on upping the capacity and longevity of the technology.